formal charge on co2|carbon dioxide formal charge : Manila Subtract the number of electrons in the circle from the group number of the element (the Roman numeral from the older system of group numbering, NOT the . bmw555 Sa 500 Slots, pati na rin ang Tongits, Sabong, Live poker at iba pang okada o Solaire na live na laro,instant withdrawal! bmw555 online casino brand ay malawak na kinikilala bilang isa sa mga pinaka-kagalang-galang na provider ng laro ng casino sa merkado ngayon.It is trending, trusted sa mga influencers. Register, bmw555 mag-log in .
formal charge on co2,537. 106K views 10 years ago. In order to calculate the formal charges for CO2 we'll use the equation Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding. Since carbon has 4 valence electrons, its formal charge will be zero. The same is true for both oxygen atoms. Both of them form 2 bonds, which means they get 2 .formal charge on co2 carbon dioxide formal charge So let me draw in the electrons in those bonds, and let's find the formal charge on carbon. The formal charge on carbon is equal to the number of valence .The formula for formal charge: F C = V-N-B 2. Here, F C is the formal charge, V is number of valence electrons, N is number of nonbonding valence electrons and B is total number . Subtract the number of electrons in the circle from the group number of the element (the Roman numeral from the older system of group numbering, NOT the .B We must calculate the formal charges on each atom to identify the more stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures .We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a . The formal charge of an atom in a molecule is the charge that would reside on the atom if all of the bonding electrons were shared equally.
We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a .
Formal Charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks .Formal charge are a way of keeping track of where the electrons an atom donates to a Lewis dot structure are placed. The sum of the formal charges equals the charge of the structure. . where one of the dots on the right side is near a carbon, meaning one of the electrons nitrogen donated is near the carbon. Exercise \(\PageIndex{1}\) Which .
Calculate the formal charge for each atom in the carbon monoxide molecule: Answer: C −1, O +1. Example 7.7. Calculating Formal Charge from Lewis Structures Assign formal charges to each atom in the interhalogen molecule BrCl 3. Solution. Step 1.In each of them, the formal charge on the center carbon is 0, the double bonded oxygen is 0, and the two single bonded oxygens are each -1. See if you can calculate these yourself correctly! Note that as discussed .

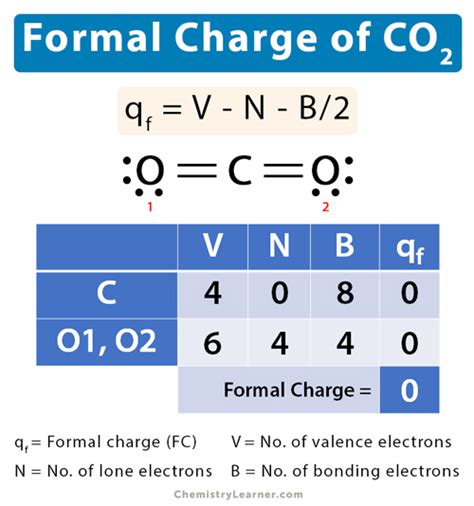
Formal charge on the Carbon atom = 4 – 0 – 8/2 = 0. ∴ The formal charge on the central Carbon (C) atom in CO2 is 0. For double-bonded Oxygen atom. Valence electrons of Oxygen = It is present in Group VI A = 6 valence electrons. Bonding electrons around Oxygen = 1 double bond = 4 electrons. Non-bonding electrons on .
The formal charges computed for the remaining atoms in this Lewis structure of carbon dioxide are shown below. It is important to keep in mind that formal charges are just that – formal, in the sense that this system is a formalism. The formal charge system is just a method to keep track of all of the valence electrons that each atom brings .
Assign formal charge. To calculate the formal charges on the atoms in CO 2, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the carbon atom, the formal charge is calculated as 4 – 0 – ½ (4) = +2, while for each oxygen atom, the formal charge is 6 – 6 – ½ (2) = -1.
In order to calculate the formal charges for CO32- we'll use the equationFormal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding e.
Thus, we calculate formal charge as follows: formal charge = # valence shell electrons (free atom) − # lone pair electrons − 1 2 # bonding electrons (3.4.1) (3.4.1) formal charge = # valence shell electrons (free atom) − # lone pair electrons − 1 2 # bonding electrons. We can double-check formal charge calculations by determining the . formal charge on carbon = (4 valence electron on isolated atom) - (0 nonbonding electrons) - (½ x 8 bonding electrons) = 4 - 0 - 4 = 0. So the formal charge on carbon is zero. For each of the hydrogens in methanol, we also get a formal charge of zero: formal charge on hydrogen = (1 valence electron on isolated atom) - (0 .Calculate the formal charges on each atom in the NH 4 + ion.. Given: chemical species Asked for: formal charges Strategy: Identify the number of valence electrons in each atom in the NH 4 + ion. Use the Lewis electron structure of NH 4 + to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation . The formal charge of carbon is 0. Step 2: Calculate the Formal Charge of Oxygen on the Left. Oxygen (O) is in group 16, so that means it has 6 valence electrons. There are 4 dots around oxygen, so .Formal charge can help us to understand the behavior of carbon monoxide, CO C O. When exposed to transition metal cations such as the iron in hemoglobin ( Fe 2+), the carbon is attracted to and binds to the . In order to calculate the formal charges for CO3 2- we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding e. So the formal charge on carbon atom is 0. So according to the formula of formal charge, you will get; Formal charge on Oxygen = Valence electrons – Nonbonding electrons – (Bonding electrons)/2 = 6 – 4 – (4/2) = 0. So the formal charge on oxygen atom is 0. Now you can see that all the atoms of CO2 have 0 formal charge.

What is a formal charge. Learn its equation, along with a few examples and diagrams. Also, learn how to find the formal charge. Chemistry Learner It's all about Chemistry. . Therefore, formal charge on carbon in CO 2 is given by, q f = 4 – 0 – 8/2 = 0. For oxygen, V = 6, N = 4, B = 4. Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO2 (carbon dioxide). In order to draw the lewis structure of CO2, first of all you have to find the total number of valence electrons present in the CO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).formal charge on co2 Using Equation 2.3.1 to calculate the formal charge on hydrogen, we obtain. Formal Charge of H = (1 valence e-) - (0 lone pair e-) - (1/2 x 2 bond pair e-) = 0. The sum of the formal charges of each atom must be equal to the overall charge of the molecule or ion.carbon dioxide formal charge Using Equation 2.3.1 to calculate the formal charge on hydrogen, we obtain. Formal Charge of H = (1 valence e-) - (0 lone pair e-) - (1/2 x 2 bond pair e-) = 0. The sum of the formal charges of each atom must be equal to the overall charge of the molecule or ion.Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge =0). Even though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all.
formal charge on co2|carbon dioxide formal charge
PH0 · formal charge of c
PH1 · carbon dioxide formal charge
PH2 · Iba pa